The minimum concentration of most raw materials that would make extraction profitable are typically several orders of magnitude higher than the average concentration within the Earth’s crust. Fortunately, geological processes in the mantle and crust can provide specific conditions that result in the enrichment of raw materials. Natural deposits of raw materials that are in large enough concentrations to be economically worthwhile to extract occur naturally throughout the Earth’s crust, but they are in discrete concentrations and must be found.
The processes that can concentrate materials include:
The resulting concentrations of any raw material that can be used by humans is termed a mineral deposit. Mineral deposits have been preserved in the rock record throughout Earth’s history (that is the past four billion years) and can be found around the globe. These active enrichment processes can be observed today on the modern seafloor, where black smoker chimneys along active tectonic plate margins are discharging hot hydrothermal fluids enriched in metals and precipitating economic deposits of lead, zinc, copper, gold and silver.

Black Smoker on the sea floor

White Island, New Zealand
Interestingly, these vent sites are also enriched in toxic elements such as mercury, arsenic, cadmium and thallium. These hydrothermal fluids (350oC) concentrate metals from the Earth’s crust in an aqueous fluid and precipitate concentrated metal minerals on the seafloor forming a mineral deposit. The process is analogous to geothermal fields in Iceland and New Zealand, where pipelines carrying hot fluids from the crust periodically become encrusted and sealed with metal sulfides and precious metals. Similarly, fumaroles on the flanks of volcanoes (e.g. White Island, New Zealand) will frequently be encrusted with deposits of native sulphur, concentrations of minerals and even precious metals.

Under unique conditions crystals can grow to very large dimensions, as in the case of the Naica Mine, Mexico.
There are three key components to any mining operation:
These three components form a production stream, where if any one of these were to stop functioning, a mine would need to cease operations.
The production of many critical metals is dependent on the production of carrier (base) metals (see Figure below). The picture below summarises the chemical and physical linkages between metals and the set of metallurgical processes that has been developed to accommodate these linkages. Base metals which are potential sources of CRMs can be seen as part of a systems-integrated metal production approach.
Metallurgical concentrates produced from mining operations need a final refining stage to produce pure metals. Elements, such as In, Ga, Ge, Te, Se and Cd among others can be electrolytically recovered at this stage to ensure the final metal produced is 99.99% pure (See Appendix A for more detail).
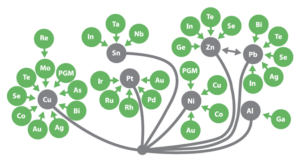
Many critical raw materials (green) are recovered as a by-product of primary base metal (grey) production.
Minerals differ from rocks in that they are a naturally occurring, homogeneous solid with an ordered internal crystalline structure which results in characteristic morphological properties.
Individual minerals have a consistent mineral formula but can vary somewhat in chemical composition due to substitution of elements with similar atomic radius and charge. In rocks, mineral grains are commonly visible with the naked eye or with the aid of a magnifying hand lens. They vary in size and can range from large centimetre sized crystals to small sub-millimetric grains visible only under a microscope. They often appear as shiny reflective surfaces, which is due to the reflection of light from mineral cleavage planes and can be a diagnostic feature for the identification of some minerals. Quite commonly, where visible, these minerals can have other diagnostic characteristics that make them easily recognizable to even inexperienced students. The more common diagnostic features are color, hardness, luster, crystal habit or shape, and cleavage. Characterization of several of these attributes in a single mineral grain is usually enough to result in the positive identification of a mineral. In addition to the type of mineral present in a rock, other conditions to be recorded include:
Since most of the major rock-forming minerals can be found in a wide range of environments, further description of their setting within the rock sample can tell much about how they came to be formed.

Form small groups and encourage students to discuss real-world problems associated with exploration and mining of resources.
Let’s think about how Toolkits can be used in a learning and teaching setting.
Use the material provided here and in the Exploration & Mining Toolkits page to develop your initial lesson.