After the input from the teacher, students should be engaged in active learning. This could be as part of the initial lesson, allowing students to develop their content knoweldge through engagement with the material. Or the activity can serve as a means to reinforce learning, extend their learning or check for understanding.
The toolkit ‘Separation of copper and iron’ contains instructions for two separate but interlinked experiments. The first involves precipitation and leaching of copper and iron. In this experiment, the principle of metal separation through a sequence of precipitation and leaching steps is illustrated with a mixture of iron and copper salts. Generally, iron and copper can be separated by altering the pH-value of an aqueous solution due to the different, but in both cases high, dependency of the solubility on the pH-value.
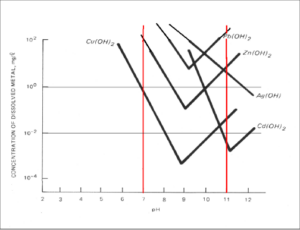
Dependency of the solubility of selected metals on pH-value.
The second experiment involves anion exchange and focusses on the ionic bonds of salts. Students have seen from the first experiment that dissolved ions can form substances different than the starting materials in chemical reactions. Anion exchangers are organic substances that can do the same thing. They contain an anion that can be exchanged for another one without altering the remaining organic molecule. In this experiment an anion exchanger, dissolved in kerosene, is used to separate iron from copper.